The Melnick lab is based in New York City at the Sandra and Edward Meyer Cancer Center of the Weill Cornell Medical College and New York Presbyterian Hospital.
About Us
The Melnick lab’s mission is to investigate the molecular underpinnings of cancer, and devise new treatments that combine lab and clinical innovations to directly benefit patients.
Our aim is to understand the mechanisms through which transcriptional and epigenetic regulation occur during normal differentiation and how these processes become disrupted in human leukemias and lymphomas and to use this information to develop specific and potent therapeutic strategies.
"Basic research is what I'm doing when I don't know what I'm doing."
〰️ Werner Von Braun 〰️
"Basic research is what I'm doing when I don't know what I'm doing." 〰️ Werner Von Braun 〰️
Recent Highlights
RECENT PUBLICATIONS
-
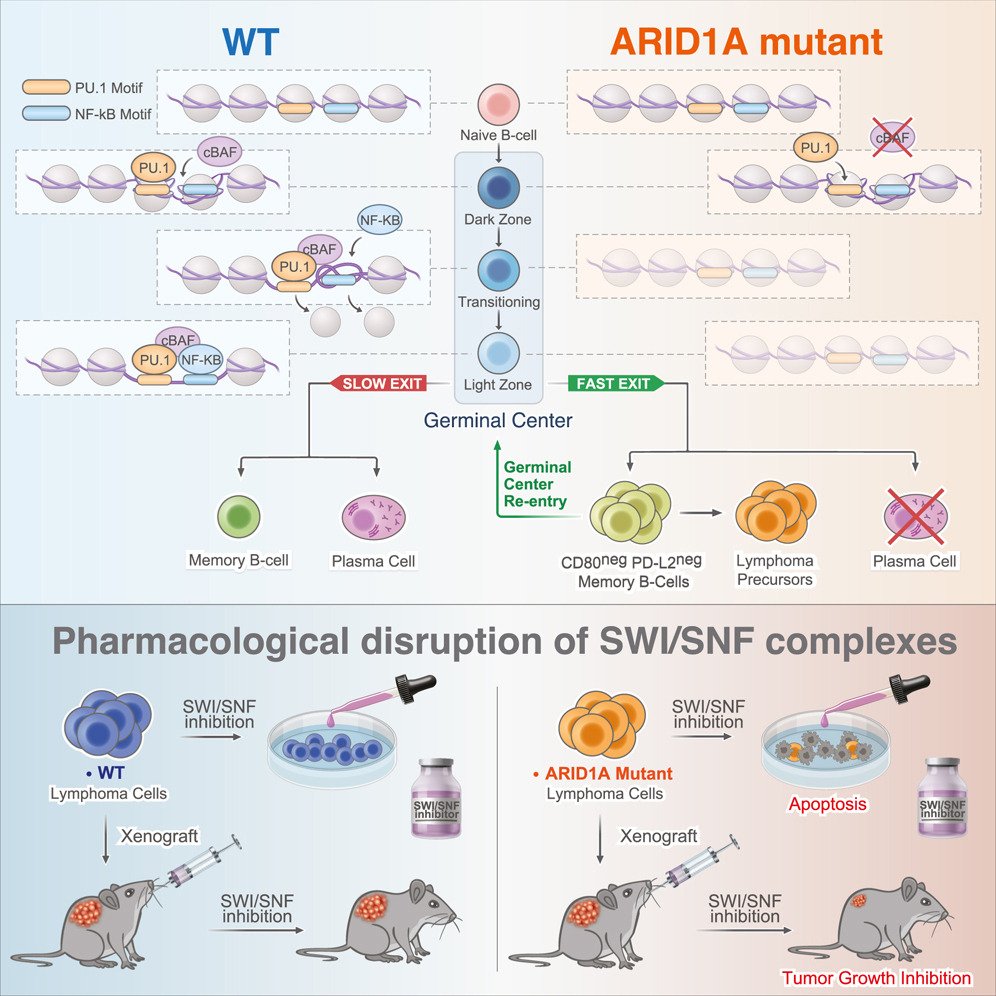
Barisic, D., Chin, C. R., Meydan, C., Teater, M., Tsialta, I., Mlynarczyk, C., Chadburn, A., Wang, X., Sarkozy, M., Xia, M., Carson, S. E., Raggiri, S., Debek, S., Pelzer, B., Durmaz, C., Deng, Q., Lakra, P., Rivas, M., Steidl, C., Scott, D. W., Weng, A. P., Mason, C. E., Green, M. R., Melnick, A. (2024). ARID1A orchestrates SWI/SNF-mediated sequential binding of transcriptional factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis. Cancer Cell. https://doi.org/10.1016/j.ccell.2024.02.010
PMID: N/A DOI: 10.1016/j.ccell.2024.02.010
-
Barisic, D., Chin, C. R., Meydan, C., Teater, M., Tsialta, I., Mlynarczyk, C., Chadburn, A., Wang, X., Sarkozy, M., Xia, M., Carson, S. E., Raggiri, S., Debek, S., Pelzer, B., Durmaz, C., Deng, Q., Lakra, P., Rivas, M., Steidl, C., Scott, D. W., Weng, A. P., Mason, C. E., Green, M. R., Melnick, A. (2024). ARID1A orchestrates SWI/SNF-mediated sequential binding of transcriptional factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis. Cancer Cell. https://doi.org/10.1016/j.ccell.2024.02.010
PMID: N/A DOI: 10.1016/j.ccell.2024.02.010
-
M Li, MR Teater, JY Hong, C Duy, H Shen, L wang, Z Chen, L Cerchietti, H Lin, A Melnick. Translational activation of ATF4 by mitochondrial anaplerotic metabolic pathways is required for DLBCL growth and survival. 2022. Blood Cancer Discovery. 3(1):50-65.
PMID: 35019856 DOI: 10.1158/2643-3230.BCD-20-0183
-
W Leung, M Teater, C Durmaz, C Meydan, AG Chivu, EJ Rice, A Muley, JM Camarillo, J Avrivalagan, A Chadburn, NL Kelleher, CG Danko, M Imielinski, SS Dave, SA Armstrong, CE Mason, KL Richards, A Melnick. SETD2 haploinsufficiency enhances germinal center-associated AICDA somatic hypermutation to drive B-cell lymphomagenesis. 2022. Cancer Discovery. 12(7):1782-1803.
PMID: 35443279 DOI: 10.1158/2159-8290.CD-21-1514
-
M Xia, L David, M Teater, J Gutierrez, X Wang, C Meydan, O Onder, K Elenitoba-Johnson, N Zamponi, L Cerchietti, T Lu, U Philippar, L Fontan, H Wu, A Melnick. BCL10 mutations define distinct dependencies guiding precision therapy for DLBCL. 2022. Cancer Discovery. 12(8):1922-1941.
PMID: 35658124 DOI: 10.1158/2159-8290.CD-21-1566
-
L Venturutti, MA Rivas, BW Pelzer, W Beguelin, R FLumann, J Hansen, M Teater, CR Chin, C Meydan, G Knittel, E Ricker, CE Mason, C Steild, DW Soctt, HR Reinhardt, AB Pernis, A Melnick. An Aged/Autoimmune B-cell Program Defines the Early Transformation of Extranodal Lymphomas. 2022. Cancer Discovery. Oct 20:CD-22-0561. doi: 10.1158/2159-8290.CD-22-0561. Online ahead of print.
PMID: 36264161 DOI: 10.1158/2159-8290.CD-22-0561
“The scientist is not a person who gives the right answers, he’s one who asks the right questions.”
-Claude Lévi-Strauss