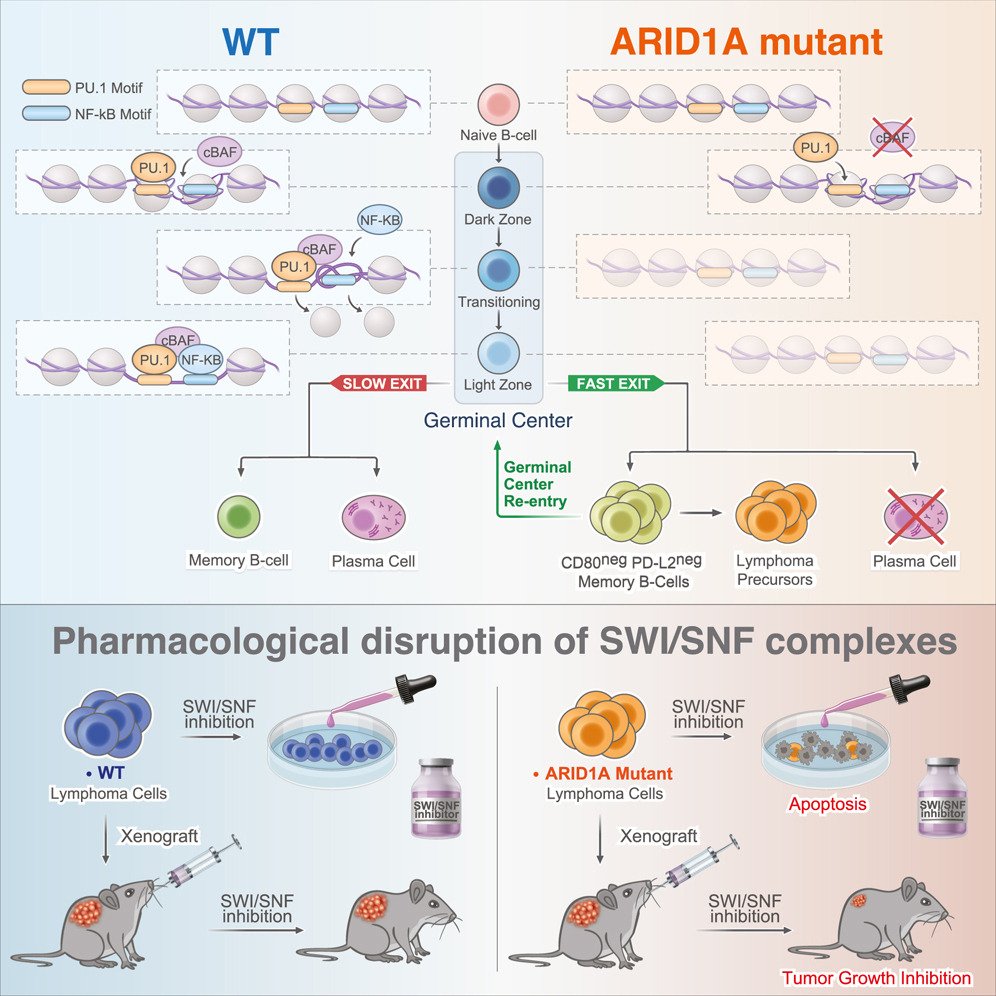
ARID1A orchestrates SWI/SNF-mediated sequential binding of transcription factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis.
Barisic, D., Chin, C. R., Meydan, C., Teater, M., Tsialta, I., Mlynarczyk, C., Chadburn, A., Wang, X., Sarkozy, M., Xia, M., Carson, S. E., Raggiri, S., Debek, S., Pelzer, B., Durmaz, C., Deng, Q., Lakra, P., Rivas, M., Steidl, C., Scott, D. W., Weng, A. P., Mason, C. E., Green, M. R., Melnick, A. (2024). ARID1A orchestrates SWI/SNF-mediated sequential binding of transcriptional factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis. Cancer Cell. https://doi.org/10.1016/j.ccell.2024.02.010
ARID1A, a subunit of the canonical BAF nucleosome remodeling complex, is commonly mutated in lymphomas. We show that ARID1A orchestrates B cell fate during the germinal center (GC) response, facilitating cooperative and sequential binding of PU.1 and NF-kB at crucial genes for cytokine and CD40 signaling. The absence of ARID1A tilts GC cell fate toward immature IgM+CD80−PD-L2− memory B cells, known for their potential to re-enter new GCs. When combined with BCL2 oncogene, ARID1A haploinsufficiency hastens the progression of aggressive follicular lymphomas (FLs) in mice. Patients with FL with ARID1A-inactivating mutations preferentially display an immature memory B cell-like state with increased transformation risk to aggressive disease. These observations offer mechanistic understanding into the emergence of both indolent and aggressive ARID1A-mutant lymphomas through the formation of immature memory-like clonal precursors. Lastly, we demonstrate that ARID1A mutation induces synthetic lethality to SMARCA2/4 inhibition, paving the way for potential precision therapy for high-risk patients.
Journal: Cancer Cell PMID: N/A DOI: 10.1016/j.ccell.2024.02.010
https://doi.org/10.1016/j.ccell.2024.02.010