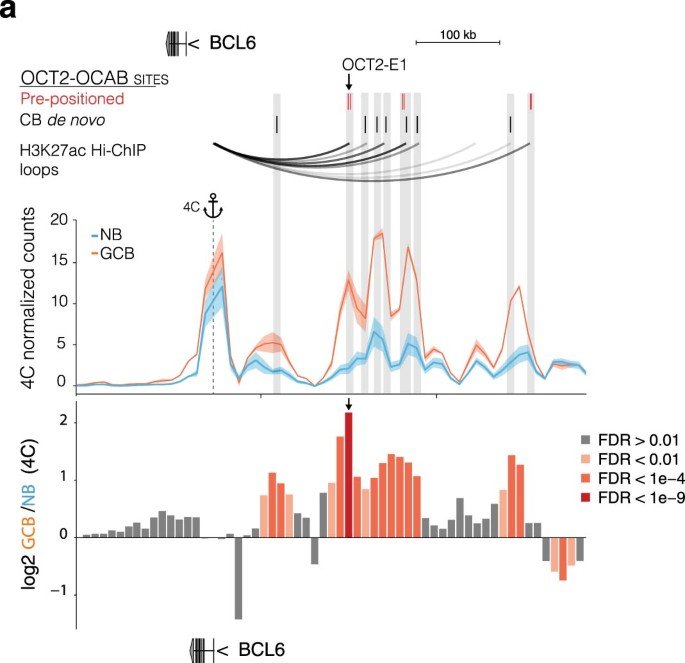
OCT2 pre-positioning facilitates cell fate transition and chromatin architecture changes in humoral immunity
During the germinal center (GC) reaction, B cells undergo profound transcriptional, epigenetic and genomic architectural changes. How such changes are established remains unknown. Mapping chromatin accessibility during the humoral immune response, we show that OCT2 was the dominant transcription factor linked to differential accessibility of GC regulatory elements. Silent chromatin regions destined to become GC-specific super-enhancers (SEs) contained pre-positioned OCT2-binding sites in naive B cells (NBs). These preloaded SE 'seeds' featured spatial clustering of regulatory elements enriched in OCT2 DNA-binding motifs that became heavily loaded with OCT2 and its GC-specific coactivator OCAB in GC B cells (GCBs). SEs with high abundance of pre-positioned OCT2 binding preferentially formed long-range chromatin contacts in GCs, to support expression of GC-specifying factors. Gain in accessibility and architectural interactivity of these regions were dependent on recruitment of OCAB. Pre-positioning key regulators at SEs may represent a broadly used strategy for facilitating rapid cell fate transitions.
Journal: Nature Immunology PMID: 34556886 DOI: 10.1038/s41590-021-01025-w
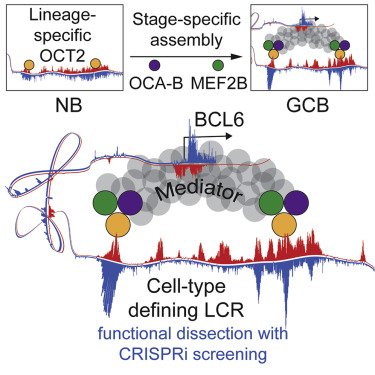
Unique Immune Cell Coactivators Specify Locus Control Region Function and Cell Stage
Locus control region (LCR) functions define cellular identity and have critical roles in diseases such as cancer, although the hierarchy of structural components and associated factors that drive functionality are incompletely understood. Here we show that OCA-B, a B cell-specific coactivator essential for germinal center (GC) formation, forms a ternary complex with the lymphoid-enriched OCT2 and GC-specific MEF2B transcription factors and that this complex occupies and activates an LCR that regulates the BCL6 proto-oncogene and is uniquely required by normal and malignant GC B cells. Mechanistically, through OCA-B-MED1 interactions, this complex is required for Mediator association with the BCL6 promoter. Densely tiled CRISPRi screening indicates that only LCR segments heavily bound by this ternary complex are essential for its function. Our results demonstrate how an intimately linked complex of lineage- and stage-specific factors converges on specific and highly essential enhancer elements to drive the function of a cell-type-defining LCR.
Journal: Molecular Cell PMID: 33232656 DOI: 10.1016/j.molcel.2020.10.036
