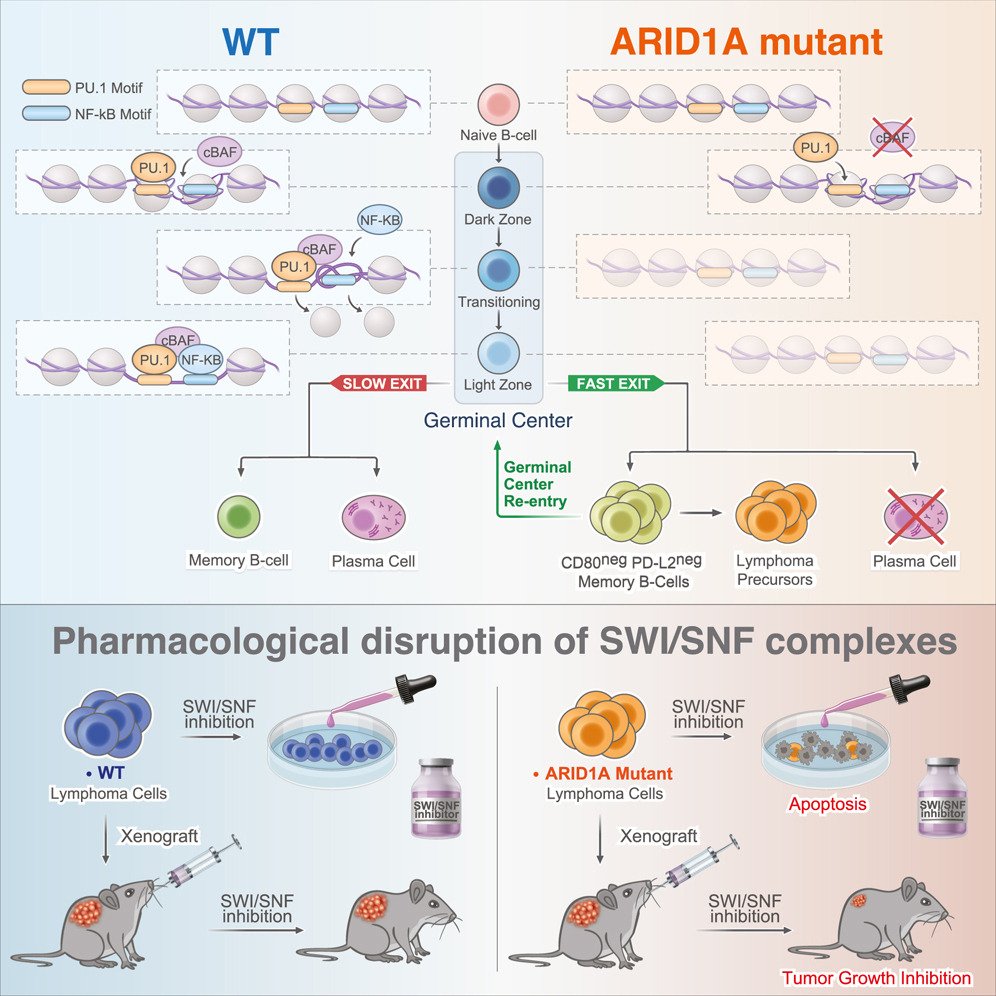
ARID1A orchestrates SWI/SNF-mediated sequential binding of transcription factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis.
ARID1A, a subunit of the canonical BAF nucleosome remodeling complex, is commonly mutated in lymphomas. We show that ARID1A orchestrates B cell fate during the germinal center (GC) response, facilitating cooperative and sequential binding of PU.1 and NF-kB at crucial genes for cytokine and CD40 signaling. The absence of ARID1A tilts GC cell fate toward immature IgM+CD80−PD-L2− memory B cells, known for their potential to re-enter new GCs. When combined with BCL2 oncogene, ARID1A haploinsufficiency hastens the progression of aggressive follicular lymphomas (FLs) in mice. Patients with FL with ARID1A-inactivating mutations preferentially display an immature memory B cell-like state with increased transformation risk to aggressive disease. These observations offer mechanistic understanding into the emergence of both indolent and aggressive ARID1A-mutant lymphomas through the formation of immature memory-like clonal precursors. Lastly, we demonstrate that ARID1A mutation induces synthetic lethality to SMARCA2/4 inhibition, paving the way for potential precision therapy for high-risk patients.
Journal: Cancer Cell PMID: N/A DOI: 10.1016/j.ccell.2024.02.010

BTG1 mutation yields supercompetitive B cells primed for malignant transformation
Multicellular life requires altruistic cooperation between cells. The adaptive immune system is a notable exception, wherein germinal center B cells compete vigorously for limiting positive selection signals. Studying primary human lymphomas and developing new mouse models, we found that mutations affecting BTG1 disrupt a critical immune gatekeeper mechanism that strictly limits B cell fitness during antibody affinity maturation. This mechanism converted germinal center B cells into supercompetitors that rapidly outstrip their normal counterparts. This effect was conferred by a small shift in MYC protein induction kinetics but resulted in aggressive invasive lymphomas, which in humans are linked to dire clinical outcomes. Our findings reveal a delicate evolutionary trade-off between natural selection of B cells to provide immunity and potentially dangerous features that recall the more competitive nature of unicellular organisms.
Journal: Science PMID: 36656933 DOI: 10.1126/science.abj7412

An Aged/Autoimmune B-cell Program Defines the Early Transformation of Extranodal Lymphomas
A third of patients with diffuse large B-cell lymphoma (DLBCL) present with extranodal dissemination, which is associated with inferior clinical outcomes. MYD88L265P is a hallmark extranodal DLBCL mutation that supports lymphoma proliferation. Yet extranodal lymphomagenesis and the role of MYD88L265P in transformation remain mostly unknown. Here, we show that B cells expressing Myd88L252P (MYD88L265P murine equivalent) activate, proliferate, and differentiate with minimal T-cell costimulation. Additionally, Myd88L252P skewed B cells toward memory fate. Unexpectedly, the transcriptional and phenotypic profiles of B cells expressing Myd88L252P, or other extranodal lymphoma founder mutations, resembled those of CD11c+T-BET+ aged/autoimmune memory B cells (AiBC). AiBC-like cells progressively accumulated in animals prone to develop lymphomas, and ablation of T-BET, the AiBC master regulator, stripped mouse and human mutant B cells of their competitive fitness. By identifying a phenotypically defined prospective lymphoma precursor population and its dependencies, our findings pave the way for the early detection of premalignant states and targeted prophylactic interventions in high-risk patients.
Significance:
Extranodal lymphomas feature a very poor prognosis. The identification of phenotypically distinguishable prospective precursor cells represents a milestone in the pursuit of earlier diagnosis, patient stratification, and prophylactic interventions. Conceptually, we found that extranodal lymphomas and autoimmune disorders harness overlapping pathogenic trajectories, suggesting these B-cell disorders develop and evolve within a spectrum.
Journal: Cancer Discovery PMID: 36264161 DOI: 10.1158/2159-8290.CD-22-0561

BCL10 Mutations Define Distinct Dependencies Guiding Precision Therapy for DLBCL
Activated B cell-like diffuse large B-cell lymphomas (ABC-DLBCL) have unfavorable outcomes and chronic activation of CARD11-BCL10-MALT1 (CBM) signal amplification complexes that form due to polymerization of BCL10 subunits, which is affected by recurrent somatic mutations in ABC-DLBCLs. Herein, we show that BCL10 mutants fall into at least two functionally distinct classes: missense mutations of the BCL10 CARD domain and truncation of its C-terminal tail. Truncating mutations abrogated a motif through which MALT1 inhibits BCL10 polymerization, trapping MALT1 in its activated filament-bound state. CARD missense mutations enhanced BCL10 filament formation, forming glutamine network structures that stabilize BCL10 filaments. Mutant forms of BCL10 were less dependent on upstream CARD11 activation and thus manifested resistance to BTK inhibitors, whereas BCL10 truncating but not CARD mutants were hypersensitive to MALT1 inhibitors. Therefore, BCL10 mutations are potential biomarkers for BTK inhibitor resistance in ABC-DLBCL, and further precision can be achieved by selecting therapy based on specific biochemical effects of distinct mutation classes.
Significance: ABC-DLBCLs feature frequent mutations of signaling mediators that converge on the CBM complex. We use structure-function approaches to reveal that BCL10 mutations fall into two distinct biochemical classes. Both classes confer resistance to BTK inhibitors, whereas BCL10 truncations confer hyperresponsiveness to MALT1 inhibitors, providing a road map for precision therapies in ABC-DLBCLs.
Journal: Cancer Discovery PMID: 35658124 DOI: 10.1158/2159-8290.CD-21-1566

SETD2 Haploinsufficiency Enhances Germinal Center–Associated AICDA Somatic Hypermutation to Drive B-cell Lymphomagenesis
SETD2 is the sole histone methyltransferase responsible for H3K36me3, with roles in splicing, transcription initiation, and DNA damage response. Homozygous disruption of SETD2 yields a tumor suppressor effect in various cancers. However, SETD2 mutation is typically heterozygous in diffuse large B-cell lymphomas. Here we show that heterozygous Setd2 deficiency results in germinal center (GC) hyperplasia and increased competitive fitness, with reduced DNA damage checkpoint activity and apoptosis, resulting in accelerated lymphomagenesis. Impaired DNA damage sensing in Setd2-haploinsufficient germinal center B (GCB) and lymphoma cells associated with increased AICDA-induced somatic hypermutation, complex structural variants, and increased translocations including those activating MYC. DNA damage was selectively increased on the nontemplate strand, and H3K36me3 loss was associated with greater RNAPII processivity and mutational burden, suggesting that SETD2-mediated H3K36me3 is required for proper sensing of cytosine deamination. Hence, Setd2 haploinsufficiency delineates a novel GCB context-specific oncogenic pathway involving defective epigenetic surveillance of AICDA-mediated effects on transcribed genes.
Significance: Our findings define a B cell-specific oncogenic effect of SETD2 heterozygous mutation, which unleashes AICDA mutagenesis of nontemplate strand DNA in the GC reaction, resulting in lymphomas with heavy mutational burden. GC-derived lymphomas did not tolerate SETD2 homozygous deletion, pointing to a novel context-specific therapeutic vulnerability.
Journal: Cancer Discovery PMID: 35443279 DOI: 10.1158/2159-8290.CD-21-1514

Translational Activation of ATF4 through Mitochondrial Anaplerotic Metabolic Pathways Is Required for DLBCL Growth and Survival
Diffuse large B-cell lymphomas (DLBCL) are broadly dependent on anaplerotic metabolism regulated by mitochondrial SIRT3. Herein we find that translational upregulation of ATF4 is coupled with anaplerotic metabolism in DLBCLs due to nutrient deprivation caused by SIRT3 driving rapid flux of glutamine into the tricarboxylic acid (TCA) cycle. SIRT3 depletion led to ATF4 downregulation and cell death, which was rescued by ectopic ATF4 expression. Mechanistically, ATF4 translation is inhibited in SIRT3-deficient cells due to the increased pools of amino acids derived from compensatory autophagy and decreased glutamine consumption by the TCA cycle. Absence of ATF4 further aggravates this state through downregulation of its target genes, including genes for amino acid biosynthesis and import. Collectively, we identify a SIRT3-ATF4 axis required to maintain survival of DLBCL cells by enabling them to optimize amino acid uptake and utilization. Targeting ATF4 translation can potentiate the cytotoxic effect of SIRT3 inhibitor to DLBCL cells. SIGNIFICANCE: We discovered the link between SIRT3 and ATF4 in DLBCL cells, which connected lymphoma amino acid metabolism with ATF4 translation via metabolic stress signals. SIRT3-ATF4 axis is required in DLBCL cells regardless of subtype, which indicates a common metabolic vulnerability in DLBCLs and can serve as a therapeutic target.
Journal: Blood Cancer Discovery PMID: 35019856 DOI: 10.1158/2643-3230.BCD-20-0183
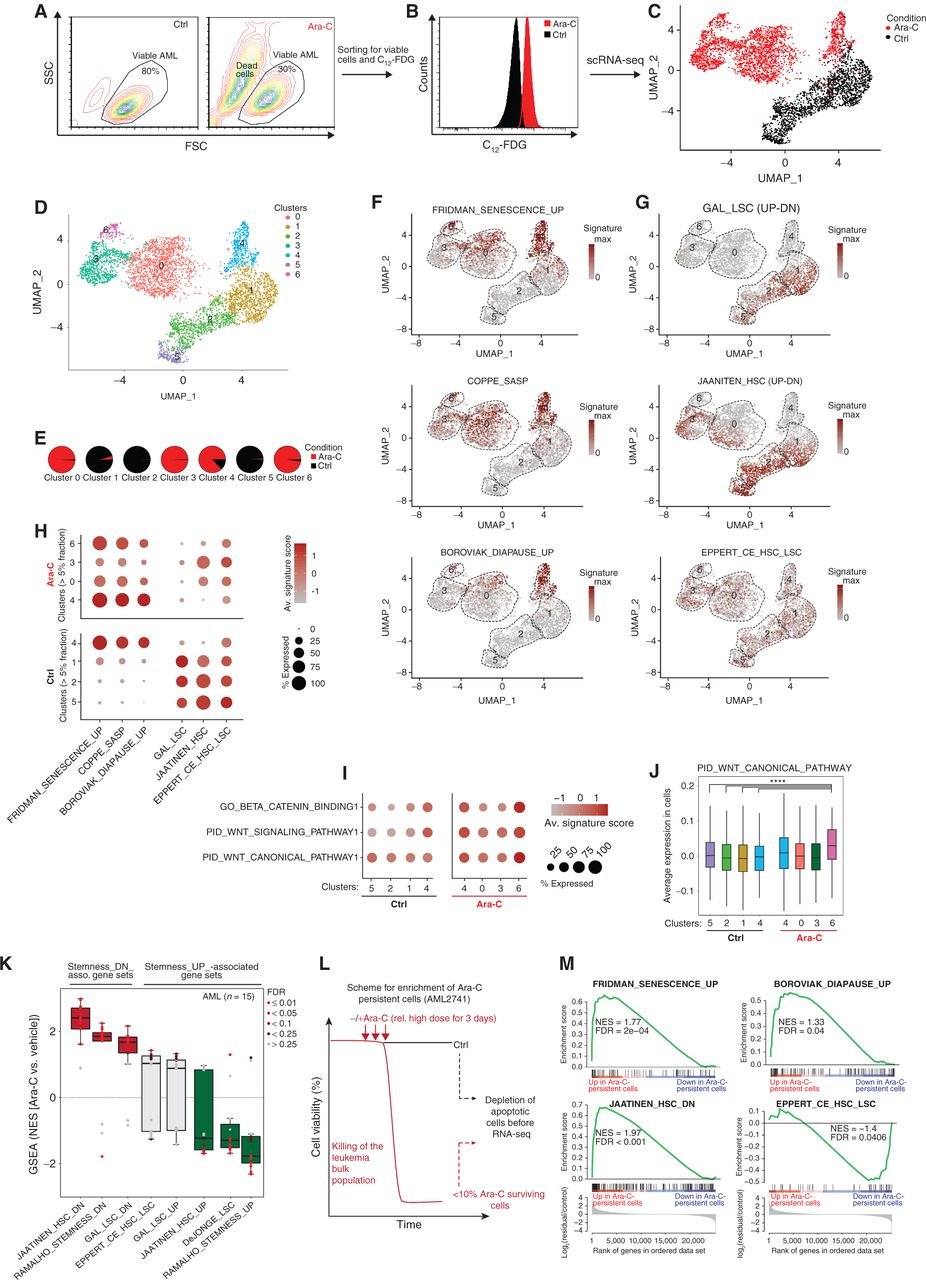
Chemotherapy Induces Senescence-Like Resilient Cells Capable of Initiating AML Recurrence
Patients with acute myeloid leukemia (AML) frequently relapse after chemotherapy, yet the mechanism by which AML reemerges is not fully understood. Herein, we show that primary AML cells enter a senescence-like phenotype following chemotherapy in vitro and in vivo. This is accompanied by induction of senescence/inflammatory and embryonic diapause transcriptional programs, with downregulation of MYC and leukemia stem cell genes. Single-cell RNA sequencing suggested depletion of leukemia stem cells in vitro and in vivo, and enrichment for subpopulations with distinct senescence-like cells. This senescence effect was transient and conferred superior colony-forming and engraftment potential. Entry into this senescence-like phenotype was dependent on ATR, and persistence of AML cells was severely impaired by ATR inhibitors. Altogether, we propose that AML relapse is facilitated by a senescence-like resilience phenotype that occurs regardless of their stem cell status. Upon recovery, these post-senescence AML cells give rise to relapsed AMLs with increased stem cell potential. SIGNIFICANCE: Despite entering complete remission after chemotherapy, relapse occurs in many patients with AML. Thus, there is an urgent need to understand the relapse mechanism in AML and the development of targeted treatments to improve outcome. Here, we identified a senescence-like resilience phenotype through which AML cells can survive and repopulate leukemia.
Journal: Cancer Discovery PMID: 33500244 DOI: 10.1158/2159-8290.CD-20-1375

Smc3 dosage regulates B cell transit through germinal centers and restricts their malignant transformation
During the germinal center (GC) reaction, B cells undergo extensive redistribution of cohesin complex and three-dimensional reorganization of their genomes. Yet, the significance of cohesin and architectural programming in the humoral immune response is unknown. Herein we report that homozygous deletion of Smc3, encoding the cohesin ATPase subunit, abrogated GC formation, while, in marked contrast, Smc3 haploinsufficiency resulted in GC hyperplasia, skewing of GC polarity and impaired plasma cell (PC) differentiation. Genome-wide chromosomal conformation and transcriptional profiling revealed defects in GC B cell terminal differentiation programs controlled by the lymphoma epigenetic tumor suppressors Tet2 and Kmt2d and failure of Smc3-haploinsufficient GC B cells to switch from B cell- to PC-defining transcription factors. Smc3 haploinsufficiency preferentially impaired the connectivity of enhancer elements controlling various lymphoma tumor suppressor genes, and, accordingly, Smc3 haploinsufficiency accelerated lymphomagenesis in mice with constitutive Bcl6 expression. Collectively, our data indicate a dose-dependent function for cohesin in humoral immunity to facilitate the B cell to PC phenotypic switch while restricting malignant transformation.
Journal: Nature Immunology PMID: 33432228 DOI: 10.1038/s41590-020-00827-8

Histone H1 loss drives lymphoma by disrupting 3D chromatin architecture
Based on gene expression profiles, diffuse large B cell lymphoma (DLBCL) is sub-divided into germinal center B cell-like (GCB) and activated B cell-like (ABC) DLBCL. Two of the most common genomic aberrations in ABC-DLBCL are mutations in MYD88, as well as BCL2 copy number gains. Here, we employ immune phenotyping, RNA-Seq and whole exome sequencing to characterize a Myd88 and Bcl2-driven mouse model of ABC-DLBCL. We show that this model resembles features of human ABC-DLBCL. We further demonstrate an actionable dependence of our murine ABC-DLBCL model on BCL2. This BCL2 dependence was also detectable in human ABC-DLBCL cell lines. Moreover, human ABC-DLBCLs displayed increased PD-L1 expression, compared to GCB-DLBCL. In vivo experiments in our ABC-DLBCL model showed that combined venetoclax and RMP1-14 significantly increased the overall survival of lymphoma bearing animals, indicating that this combination may be a viable option for selected human ABC-DLBCL cases harboring MYD88 and BCL2 aberrations.
Journal: Nature PMID: 33299181 DOI: 10.1038/s41586-020-3017-y
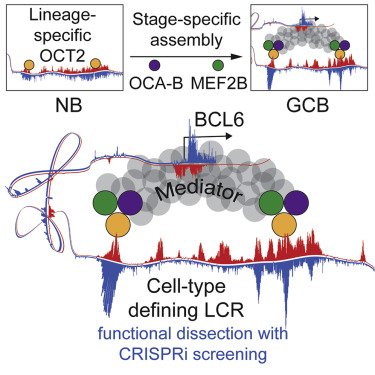
Unique Immune Cell Coactivators Specify Locus Control Region Function and Cell Stage
Locus control region (LCR) functions define cellular identity and have critical roles in diseases such as cancer, although the hierarchy of structural components and associated factors that drive functionality are incompletely understood. Here we show that OCA-B, a B cell-specific coactivator essential for germinal center (GC) formation, forms a ternary complex with the lymphoid-enriched OCT2 and GC-specific MEF2B transcription factors and that this complex occupies and activates an LCR that regulates the BCL6 proto-oncogene and is uniquely required by normal and malignant GC B cells. Mechanistically, through OCA-B-MED1 interactions, this complex is required for Mediator association with the BCL6 promoter. Densely tiled CRISPRi screening indicates that only LCR segments heavily bound by this ternary complex are essential for its function. Our results demonstrate how an intimately linked complex of lineage- and stage-specific factors converges on specific and highly essential enhancer elements to drive the function of a cell-type-defining LCR.
Journal: Molecular Cell PMID: 33232656 DOI: 10.1016/j.molcel.2020.10.036

TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate
The most aggressive B cell lymphomas frequently manifest extranodal distribution and carry somatic mutations in the poorly characterized gene TBL1XR1. Here, we show that TBL1XR1 mutations skew the humoral immune response toward generating abnormal immature memory B cells (MB), while impairing plasma cell differentiation. At the molecular level, TBL1XR1 mutants co-opt SMRT/HDAC3 repressor complexes toward binding the MB cell transcription factor (TF) BACH2 at the expense of the germinal center (GC) TF BCL6, leading to pre-memory transcriptional reprogramming and cell-fate bias. Upon antigen recall, TBL1XR1 mutant MB cells fail to differentiate into plasma cells and instead preferentially reenter new GC reactions, providing evidence for a cyclic reentry lymphomagenesis mechanism. Ultimately, TBL1XR1 alterations lead to a striking extranodal immunoblastic lymphoma phenotype that mimics the human disease. Both human and murine lymphomas feature expanded MB-like cell populations, consistent with a MB-cell origin and delineating an unforeseen pathway for malignant transformation of the immune system.
Journal: Cell PMID: 32619424 DOI: 10.1016/j.cell.2020.05.049

Mutant EZH2 Induces a Pre-malignant Lymphoma Niche by Reprogramming the Immune Response
Follicular lymphomas (FLs) are slow-growing, indolent tumors containing extensive follicular dendritic cell (FDC) networks and recurrent EZH2 gain-of-function mutations. Paradoxically, FLs originate from highly proliferative germinal center (GC) B cells with proliferation strictly dependent on interactions with T follicular helper cells. Herein, we show that EZH2 mutations initiate FL by attenuating GC B cell requirement for T cell help and driving slow expansion of GC centrocytes that become enmeshed with and dependent on FDCs. By impairing T cell help, mutant EZH2 prevents induction of proliferative MYC programs. Thus, EZH2 mutation fosters malignant transformation by epigenetically reprograming B cells to form an aberrant immunological niche that reflects characteristic features of human FLs, explaining how indolent tumors arise from GC B cells.
Journal: Cancer Cell PMID: 32396861 DOI: 10.1016/j.ccell.2020.04.004

Non-oncogene Addiction to SIRT3 Plays a Critical Role in Lymphomagenesis
Diffuse large B cell lymphomas (DLBCLs) are genetically heterogeneous and highly proliferative neoplasms derived from germinal center (GC) B cells. Here, we show that DLBCLs are dependent on mitochondrial lysine deacetylase SIRT3 for proliferation, survival, self-renewal, and tumor growth in vivo regardless of disease subtype and genetics. SIRT3 knockout attenuated B cell lymphomagenesis in VavP-Bcl2 mice without affecting normal GC formation. Mechanistically, SIRT3 depletion impaired glutamine flux to the TCA cycle via glutamate dehydrogenase and reduction in acetyl-CoA pools, which in turn induce autophagy and cell death. We developed a mitochondrial-targeted class I sirtuin inhibitor, YC8-02, which phenocopied the effects of SIRT3 depletion and killed DLBCL cells. SIRT3 is thus a metabolic non-oncogene addiction and therapeutic target for DLBCLs.
Journal: Cancer Cell PMID: 31185214 DOI: 10.1016/j.ccell.2019.05.002

Rational Targeting of Cooperating Layers of the Epigenome Yields Enhanced Therapeutic Efficacy against AML
Disruption of epigenetic regulation is a hallmark of acute myeloid leukemia (AML), but epigenetic therapy is complicated by the complexity of the epigenome. Herein, we developed a long-term primary AML ex vivo platform to determine whether targeting different epigenetic layers with 5-azacytidine and LSD1 inhibitors would yield improved efficacy. This combination was most effective in TET2mut AML, where it extinguished leukemia stem cells and particularly induced genes with both LSD1-bound enhancers and cytosine-methylated promoters. Functional studies indicated that derepression of genes such as GATA2 contributes to drug efficacy. Mechanistically, combination therapy increased enhancer–promoter looping and chromatin-activating marks at the GATA2 locus. CRISPRi of the LSD1-bound enhancer in patient-derived TET2mut AML was associated with dampening of therapeutic GATA2 induction. TET2 knockdown in human hematopoietic stem/progenitor cells induced loss of enhancer 5-hydroxymethylation and facilitated LSD1-mediated enhancer inactivation. Our data provide a basis for rational targeting of cooperating aberrant promoter and enhancer epigenetic marks driven by mutant epigenetic modifiers.
Journal: Cancer Discovery PMID: 31076479 DOI: 10.1158/2159-8290.CD-19-0106
