TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate
Venturutti, L., Teater, M., Zhai, A., Chadburn, A., Babiker, L., Kim, D., Béguelin, W., Lee, T. C., Kim, Y., Chin, C. R., Yewdell, W. T., Raught, B., Phillip, J. M., Jiang, Y., Staudt, L. M., Green, M. R., Chaudhuri, J., Elemento, O., Farinha, P., Weng, A. P., … Melnick, A. M. (2020). TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate. Cell, 182(2), 297–316.e27. https://doi.org/10.1016/j.cell.2020.05.049
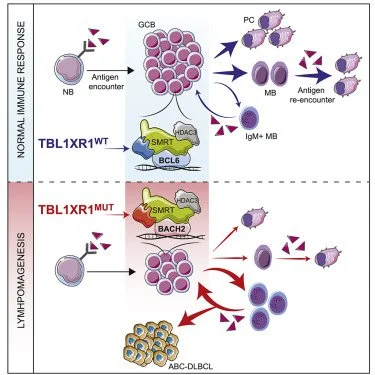
The most aggressive B cell lymphomas frequently manifest extranodal distribution and carry somatic mutations in the poorly characterized gene TBL1XR1. Here, we show that TBL1XR1 mutations skew the humoral immune response toward generating abnormal immature memory B cells (MB), while impairing plasma cell differentiation. At the molecular level, TBL1XR1 mutants co-opt SMRT/HDAC3 repressor complexes toward binding the MB cell transcription factor (TF) BACH2 at the expense of the germinal center (GC) TF BCL6, leading to pre-memory transcriptional reprogramming and cell-fate bias. Upon antigen recall, TBL1XR1 mutant MB cells fail to differentiate into plasma cells and instead preferentially reenter new GC reactions, providing evidence for a cyclic reentry lymphomagenesis mechanism. Ultimately, TBL1XR1 alterations lead to a striking extranodal immunoblastic lymphoma phenotype that mimics the human disease. Both human and murine lymphomas feature expanded MB-like cell populations, consistent with a MB-cell origin and delineating an unforeseen pathway for malignant transformation of the immune system.
Journal: Cell PMID: 32619424 DOI: 10.1016/j.cell.2020.05.049